This pilot clinical study investigates the impact of human fasting on macrophage function and lifespan in Caenorhabditis elegans by modulating multiple bioactive metabolites, a combination of spermidine, and three other ingredients.
This study examined the effects of prolonged fasting (PF) on metabolic and immune health in humans. The key points of the study are summarized below:
- Assessments of four distinct metabolic states were assessed, and metabolomic profiling of participant plasma was used to identify bioactive metabolites.
- Fasting was observed to modulate the plasma metabolome and confer beneficial immunomodulatory effects on human macrophages.
- Four bioactive metabolites upregulated during PF (spermidine, 1-methylnicotinamide, palmitoylethanolamide, and oleoylethanolamide) could replicate these immunomodulatory effects and extend median lifespan of C. elegans by as much as 96%. The findings provide insight into functionalities, immunological pathways, fasting mimetic compounds, and targets for longevity research.
Introduction
Studies suggest that periodic prolonged fasting, which involves abstaining from caloric intake for 24 hours, may provide significant health benefits beyond lifespan extension. Such effects could include reducing inflammation-related diseases and metabolic disorders by altering cellular processes related to aging. According to reviewed studies on this topic, these results are likely due to the immunomodulatory properties exerted on innate and adaptive immune systems through PF practices.
In innate immunity, PF has been shown to affect macrophage polarization, inflammatory signaling pathways, and monocyte-dependent autoimmune disease progression without impairing appropriate responses to infection or cancer.
The innate immune system is indispensable in preserving overall health and longevity, as uncontrolled inflammation can take a toll on our bodies, leading to chronic conditions like neurodegenerative diseases. Its proper regulation could be the key to maintaining optimal well-being even with advancing age.
Despite our understanding of the relationship between PF and immunomodulation, further research is needed to investigate how this occurs at the molecular level. Eating has been shown to interfere with immune homeostasis by inducing proinflammatory effects in everyone – suggesting that consuming food too often could lead to chronic inflammation.
The traditional emphasis on diet quality had overshadowed an even more fundamental element of health and wellbeing – meal timing. Yet, its significance is finally being recognized with recent research into the potential benefits of intermittent fasting.
Research has uncovered the biology behind fasting and its potential to prevent or treat immune system disorders. While prolonged periods between meals can benefit some, it is not recommended for individuals with underlying health conditions who cannot fast. Identifying molecular mediators in periodic fasting could pave a pathway towards developing compounds that allow those restricted from traditional fasting methods to still reap the same rewards without having to change their lifestyle drastically.
The pilot clinical trial was designed to explore the response of young and healthy participants when subjected to 36 hours of fasting. Four nutrition states were evaluated to examine potential molecular mediators that impact plasma metabolome and macrophage functionalities.
Methods
The detailed methods are in the PDF file here. Below is the outline of the study methods:
- Human clinical trial of Periodic prolonged fasting
- Blood Processing
- NMR Lipo Profile
- Metabolomic Analyses
- Primary Macrophage Isolation
- THP-1 Macrophage Differentiation
- Functional Analyses of Participant Plasma and Isolated Metabolites
- Caenorhabditis Elegans Lifespan Studies
- Statistical Analysis
Results
- PF significantly alters clinical markers of metabolic health
- PF enhances plasma functionalities and induces anti-inflammatory effects in macrophage
- PF robustly and uniquely alters the human plasma metabolome
- PF upregulates circulation of multiple immunomodulatory metabolites
- Fasting metabolites replicate the anti-inflammatory effects of fasted plasma in macrophage
- Fasting metabolites extend lifespan in C. elegans
Fasting metabolites extend lifespan in C. elegans
Beyond immunomodulation, PF has also been shown to significantly extend the lifespan in model organisms. To assess the potential involvement of spermidine, 1-MNA, PEA, and OEA in mediating the lifespan-extending effects of PF, we performed lifespan analyses of C. elegans whose medium contained individual metabolites or their combination. Strikingly, we found that treatment with spermidine, PEA, OEA, and the combination of spermidine, PEA, OEA, and 1-MNA (combo), but not 1-MNA alone, showed significantly increased lifespan extension versus untreated control worms. The hazard ratios (HRs) (and 95% confidence intervals [CIs]) for each treatment group versus the control were as follows: 1) spermidine, HR, 0.672 (95% CI: 0.498, 0.907); 2) 1-MNA, HR, 1.061 (95% CI: 0.789, 1.426); 3) PEA, HR, 0.362 (95% CI: 0.265, 0.493); 4) OEA, HR, 0.418 (95% CI: 0.304, 0.575); and 5) combo, HR, 0.147 (95% CI: 0.107, 0.202). Of the individual metabolites, PEA and OEA were found to have the most significant effect on lifespan extension at the lowest concentrations and significantly increased lifespan versus
Combination of spermidine, PEA, OEA and 1-MNA
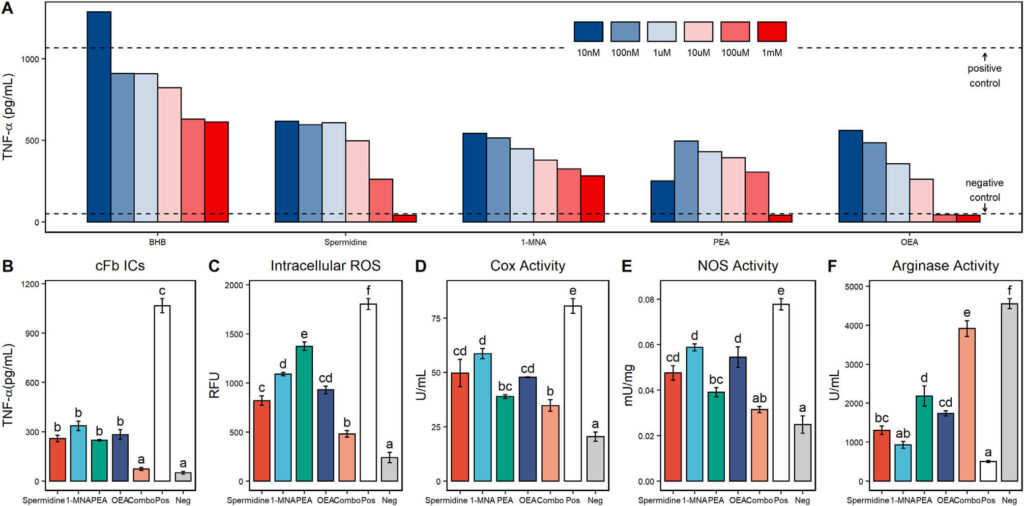
Figure 4. Metabolites upregulated during fasting induce anti-inflammatory effects in macrophages. Tumor necrosis factor alpha (TNF-α) secretion from citrullinated fibrinogen immune complex (cFb IC)-stimulated primary human macrophage treated with beta-hydroxybutyrate (BHB), spermidine, 1-methylnicotinamide (1-MNA), palmitoylethanolamide (PEA), and oleoylethanolamide (OEA) at a final concentration of 1–10 nM (A). The TNF-α levels from unstimulated macrophage are shown as negative control values, and the TNF-α levels from stimulated macrophage without any other treatment are shown as positive control values. TNF-α secretion from cFb IC-stimulated primary human macrophage treated with spermidine (100 μM), 1-MNA (100 μM), PEA (10 μM), OEA (10 μM), and a combination treatment (combo) of all 4 metabolites at their individual concentrations (B).
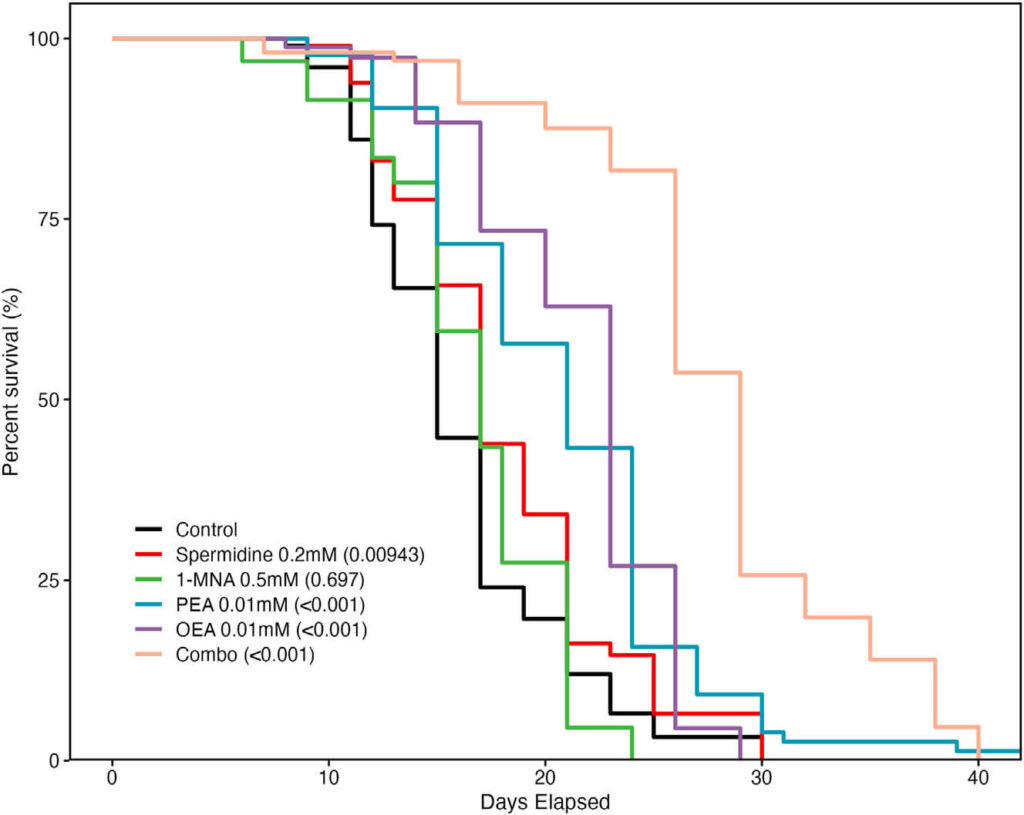
Figure 5. Metabolites upregulated during fasting extend lifespan in Caenorhabditis elegans: lifespan analysis of C. elegans with either no treatment (control) or lifelong exposure to spermidine (200 μM), 1-MNA (500 μM), PEA (10 μM), OEA (10 μM), or a combination of all 4 metabolites (combo).
Discussion
The pilot clinical study demonstrated the remarkable power of PF – a fasting-induced alteration in human plasma. Fasted macrophages underwent an anti-inflammatory effect likely mediated by specific upregulated bioactive metabolites after 36 hours of fasting, which even extended lifespan when studied with C. elegans organisms.
This study was distinguished by its stringent and meticulous design. Crucially, individual dietary intake was controlled while compliance to fasting practices was monitored via personal glucose readings. This enabled us to compare four distinct nutritional statuses – a 36-h fasted state versus an overnight fasted state, as well as postprandial states in both before and after the 36 h of fasting period. These measures provide greater detail than other trials on prolonged periods of fasting typically yield.
This research demonstrated that Prolonged Fasting (PF) could have distinct effects beyond those achieved during a typical overnight fast and maintain its benefits into the re-fed state without diet. Results showed PF capable of increasing plasma antioxidant capacity and cholesterol efflux ability over baseline – with these increases carrying on to post fasting period. Furthermore, treatment of nonfasted macrophages by fasting participants’ plasma induced potent anti-inflammatory activity, decreasing TNF-α secretion, intracellular ROS accumulation, and M1 polarization responses while lowering total COX activity in human macrophages compared to fed or preformed states.
These studies break new ground in understanding human plasma functionalities – showing that prolonged fasting (PF) has beneficial effects, inducing significant anti-inflammatory activity and ameliorating Fc-γ receptor-induced macrophage activation. This is the first report on PF’s ability to decrease M1 polarization responses amongst humans acutely. Our work further elucidates molecular pathways involved in immunomodulation by PF entering previously uncharted territory.
By treating primary cells with participants’ plasma during in vitro analyses, these studies offer insight into the systemic and cellular consequences of PF in humans. Allowing for sensitive and real-time readouts to be captured throughout a clinical study, this ex vivo modeling method provides an invaluable way to examine molecular mechanisms behind such effects thoroughly.
Results demonstrate that certain anti-inflammatory benefits of mimicking a fasted state with plasma are achievable in nonfasting cells. This suggests there may be multiple elements to fasting’s effect, some of which operate independently from the body’s energy balance.
Investigating possible plasma-borne mediators of the beneficial effects of periodic fasting (PF) could be hugely impactful, potentially leading to therapeutic or preventative interventions for health and disease. In our investigations into these mediators, we discovered that a remarkable alteration had occurred in the human metabolome between fasted state versus baseline – with 389 metabolites found significantly different upon comparison. Unlocking this potential offers an exciting prospect.
Selected metabolites that have more potent anti-inflammatory effects in their fasted state, spermidine, 1-MNA, PEA, and OEA outperformed the traditionally known immunomodulatory metabolite BHB. Moreover, their analyzed performance showed considerable potential for inflammatory responses beyond previously thought possible.
An extensive assessment of various metabolites and combinations identified significant anti-inflammatory effects in fasting plasma, including reductions to TNF-α secretion, oxidative stress levels, cyclooxygenase activity rate, and M1 polarization responses. Notably, the combination showed a significant decline than the individual components alone, signifying that the upregulation of these elements is responsible for fasted state’s beneficial impact on inflammation.
In this groundbreaking trial, four metabolites – spermidine, 1-MNA, PEA and OEA – were upregulated in humans during fasting. This combination of these compounds can replicate the functional effects of Fasting with nonfasted cells. Additionally, this study is pioneering as it shows that when combined, their immunomodulatory abilities far exceed each metabolite’s individual effect on human immunity.
Our findings demonstrate that lifelong treatment of C. elegans with spermidine, PEA, OEA, and especially the combined mixture of all four compounds prolongs life expectancy beyond what is normally seen in control worms even under standard feeding conditions. Previous research has highlighted how spermidine and 1-MNA can induce autophagy or alter ROS production to increase longevity, respectively – now we show this combination leads to an impressive lifespan extension in these fascinating creatures.
PEA and OEA hold the potential to significantly extend lifespan, with median extension reaching up to 96%. In humans, this effect is thought to be related to innate immune system activity – similarly observed for longevity in worms. Investigation into these compounds suggests their influence on multiple cellular processes works synergistically to extend lifespans.
The results have signaled the crucial role of metabolites in life extension through PF, and their potential use as fasting mimetics. This groundbreaking study demonstrates how PEA and OEA can positively influence longevity- thus revealing two novel molecules that should be further explored for lifespan studies.
This groundbreaking trial uncovered several promising molecules and pathways that may be instrumental in developing interventions to replicate the effects of periodic fasting. It was remarkable how PEA and OEA were found to have an unexpected influence on extending lifespan, providing a key insight for further investigation into longevity treatments. Overall, this study provides compelling evidence for using human-based approaches when seeking healthful ingredients with therapeutic potential – unlocking exciting possibilities for future efforts toward promoting long-term wellbeing.
Uncovering how bioactive molecules impact longevity in humans and other model organisms could significantly accelerate the discovery of age-defying targets directly applicable to human wellbeing. While spermidine has already been explored as a potential caloric restriction mimetic, further study is needed into 1-MNA, PEA, and OEA – not forgetting synergistic effects between different fasting or calorie control mimics.
Clinical trials are needed to explore combining spermidine, 1-MNA, PEA, and OEA as an alternative method for mimicking fasting’s effects. Future studies should aim to assess their effectiveness and practicality.
Literature References:
- Rhodes CH, Zhu C, Agus J, Tang X, Li Q, Engebrecht J, Zivkovic AM. Human fasting modulates macrophage function and upregulates multiple bioactive metabolites that extend lifespan in Caenorhabditis elegans: a pilot clinical study. Am J Clin Nutr. 2022 Dec 20:S0002-9165(22)10526-5. doi: 10.1016/j.ajcnut.2022.10.015IF: 8.472 Q1 B1. Epub ahead of print. PMID: 36811567IF: 8.472 Q1 B1.