Although aging is inevitable, many supplements like NMN (Nicotinamide mononucleotide) can help in boosting NAD+ levels in the body. NMN is an NAD+ precursor that can enhance NAD+ levels in every living organism. NAD+ has the ability to control a wide range of biological processes, including mitochondrial activity, DNA repair, chromosome integrity, gene expression, and axonal integrity and regeneration. NMN is shown to be relevant in the therapeutic domain in recent preclinical trials. Realizing how important this compound is in preventing aging and maintaining the aging process, it became a crucial key. NMN has been discovered to be extremely effective in treating other age and health-related disorders by increasing NAD+ levels.
The key findings
To evaluate how NMN can increase NAD+ levels, this study uses the NMN precursor, called MIB-626. For its superior therapeutic qualities in a variety of human disorders, including Alzheimer’s, dementia, and other age-related conditions, MIB-626, or a microcrystalline unique polymorph NMN formulation, has gained attention in new studies. The preclinical study supports MIB-626’s potential therapeutic effects.
For this research, a total of 30 overweight/obese participants aged 45 and above were given 1g of MIB-626 or a placebo twice daily for 28 days without changing their food or lifestyle. The results from this study were focused on safety, blood pressure, lipids, athletic performance, body weight, liver fat, insulin sensitivity, and muscle metabolism.
Between groups, side effects were minimal. The MIB-626 therapy significantly boosted the levels of NAD and its metabolites in the blood. Total cholesterol, LDL, and other parameters were reduced dramatically more in the MIB-626 group than in the placebo group. There were also differences in body weight, and blood pressure. There were no significant changes between the groups in muscle strength, aerobic capacity, insulin sensitivity, and hepatic and intra-abdominal fat. When the administration was terminated after 28 days, NAD+ and its metabolites normalized to the level of the control group after 56 days.
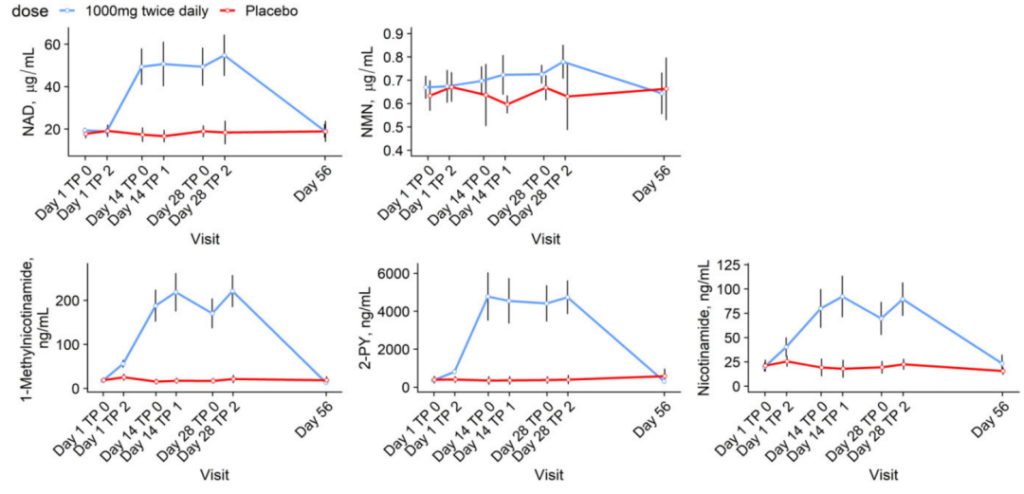
Pencina KM, Valderrabano R, Wipper B, Orkaby AR, Reid KF, Storer T, Lin AP, Merugumala S, Wilson L, Latham N, Ghattas-Puylara C, Ozimek NE, Cheng M, Bhargava A, Memish-Beleva Y, Lawney B, Lavu S, Swain PM, Apte RS, Sinclair DA, Livingston D, Bhasin S. Nicotinamide Adenine Dinucleotide Augmentation in Overweight or Obese Middle-Aged and Older Adults: A Physiologic Study. J Clin Endocrinol Metab. 2023 Feb 6:dgad027. doi: 10.1210/clinem/dgad027. Epub ahead of print. PMID: 36740954.
(After 56 days, NAD+ and its metabolites returned to normal levels when the treatment after 28 days was stopped.)
Additionally, the participants who received MIB-626 experienced significant weight loss and enhanced total cholesterol, LDL, and HDL levels. The muscle mass and physical function slightly improved as well as the blood pressure, but not too much.
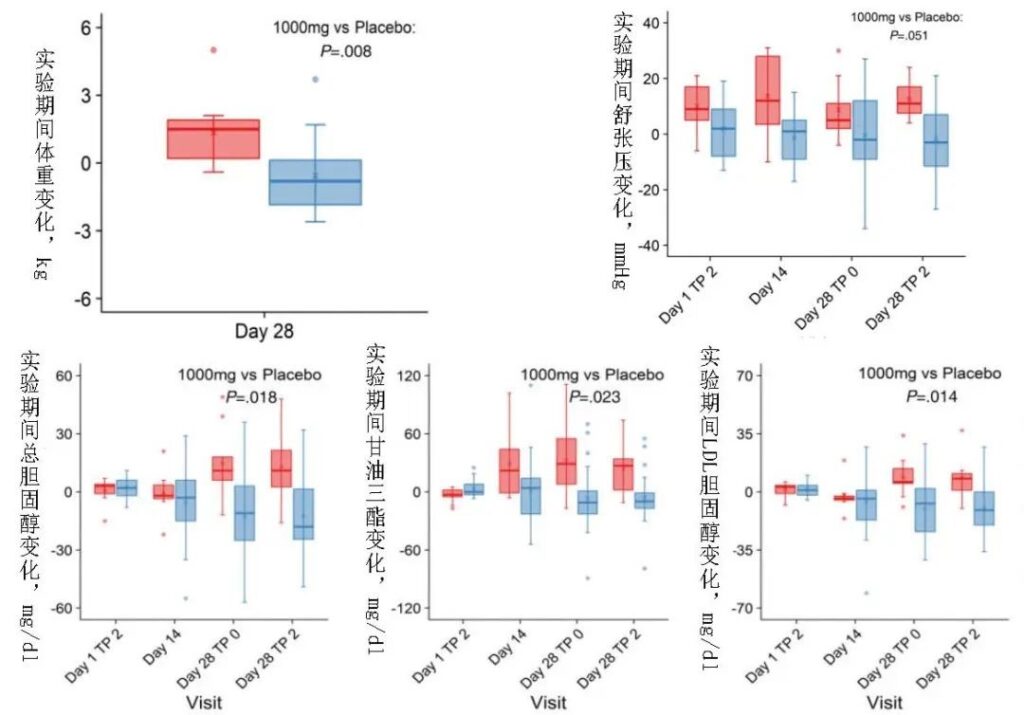
Pencina KM, Valderrabano R, Wipper B, Orkaby AR, Reid KF, Storer T, Lin AP, Merugumala S, Wilson L, Latham N, Ghattas-Puylara C, Ozimek NE, Cheng M, Bhargava A, Memish-Beleva Y, Lawney B, Lavu S, Swain PM, Apte RS, Sinclair DA, Livingston D, Bhasin S. Nicotinamide Adenine Dinucleotide Augmentation in Overweight or Obese Middle-Aged and Older Adults: A Physiologic Study. J Clin Endocrinol Metab. 2023 Feb 6:dgad027. doi: 10.1210/clinem/dgad027. Epub ahead of print. PMID: 36740954.
(Changes in the body weight, blood lipids, and blood pressure in the MIB-626 group.)
Limitations in the study
Although 30 participants were part of this trial, there were actually fewer. There were initially 24 people enrolled, but since 6 of them withdrew from the trial for different reasons, the final number was less than 30. Moreover, in this 28-day trial, the researchers only examined the participants’ indicators on 3 points including the first, fourteenth, and twenty-eighth days, which made it impossible to demonstrate the patients’ multiple symptoms more clearly. There is insufficient time to properly confirm whether there are variations in insulin sensitivity and glucose metabolism.
Despite being a randomized trial, the average age of the participants in the evaluation was 60 years old and of the placebo group was 63 years old, which makes it controversial to the final results in anti-aging diseases. Furthermore, all participants in the study were obese and older people. As a result, it is unclear if MIB-626 can be taken in generally healthy individuals.
Although the results were positive, and there were small to no adverse effects of using MIB-626, this clinical study requires numerous improvements in order to be sure that this supplement can be taken in healthy and younger people, as well as present positive benefits in other parameters.
Previous MIB-626 clinical trials
MIB-626 had positive benefits in previous studies. In one double-blind study, the results show good MIB-626 tolerance with no negative effects, when given for 14 days. The participants received a placebo, the present dosage once daily, and twice daily. MIB-626 treatment resulted in considerably elevated levels of NAD and NAD metabolites that were dose-dependent. A safe dosage of MIB-626 was thought to be 1000mg administered once or twice daily.
Another clinical trial was done for treating Friedreich’s Ataxia (FA) in adults. The safety and tolerability of short-term therapy were the main objectives of this experiment. Two 500 mg MIB-626 tablets were used orally each day during this trial in only one group, without the placebo group. The study is still ongoing.
Currently, professor David Sinclar is concentrating on the MIB-626 experimental therapy. MIB-626 possesses anti-inflammatory and immunomodulatory effects. For years, David Sinclar and his colleagues have been developing this drug and testing it on older people. Positive feedback was found in the studies’ final outcomes.
Conclusion
MIB-626 treatment enhances NAD+ levels in obese, elderly, and middle-aged people, while also reducing total cholesterol, LDL and HDL levels, body weight, and blood pressure with no side effects. These findings support the need for larger studies to determine whether increasing NAD is effective in enhancing cardiometabolic outcomes in aged people. In addition, there were some limitations to these findings, so further evaluation is needed to determine the MIB-626 supplementation in healthy people.
References:
- Pencina KM, Valderrabano R, Wipper B, Orkaby AR, Reid KF, Storer T, Lin AP, Merugumala S, Wilson L, Latham N, Ghattas-Puylara C, Ozimek NE, Cheng M, Bhargava A, Memish-Beleva Y, Lawney B, Lavu S, Swain PM, Apte RS, Sinclair DA, Livingston D, Bhasin S. Nicotinamide Adenine Dinucleotide Augmentation in Overweight or Obese Middle-Aged and Older Adults: A Physiologic Study. J Clin Endocrinol Metab. 2023 Feb 6:dgad027. doi: 10.1210/clinem/dgad027. Epub ahead of print. PMID: 36740954